Microcanonical ensemble
Microcanonical ensemble
- It is a collection of essentially independent assemblies having the same energy E, volume V, and number of system N.
- All the systems are of same type.
- The independent assemblies are separated by rigid, impermeable and well insulated walls such that the values of E, V and N are not affected by the presence of other system.
- We can not actually specify the macroscopic energy of an assembly exactly.

- We consider two neighbouring surfaces with energies E and E + ΔE.
- In macroscopic ensemble every system has N molecules, V volume and energy between E and E + ΔE
- Also the average value of total momentum of the system = 0

- Let Γ(E) = Volume in Γ-space occupied by the microcanonical ensemble.
- Let Σ(E) = Volume in Γ-space enclosed by the energy surface of energy E
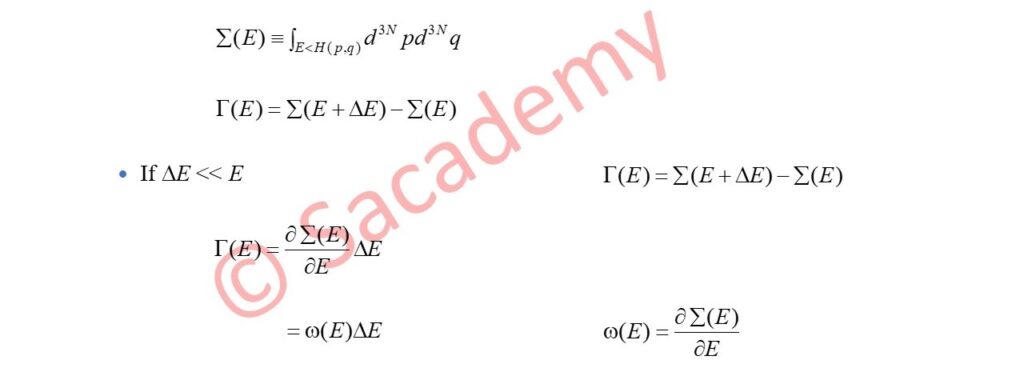
- Here ω(E) = Density of the system at the energy E.
- If S = Entropy of the system, and k = Boltzmann’s constant, then.
S (E, V) = k log Γ (E)
S is an extensive property
- If a system is composed of two sufficient large sub-systems having entropies S1 and S2, then the entropy of the total system is
S = S1 + S2
- Let a system be composed of two sub-systems having N1 and N2 particles in the subsystems and V1 and V2 be the volumes corresponding to these subsystems respectively.
- If H1 (p1, q1) and H2 (p2, q2) are the Hamiltonian of the two subsystems then the total Hamiltonian of the composite system is
H (p, q) = H1 (p1, q1) + H2 (p2, q2)
- Let the two subsystems be isolated from each other
- The energy of first subsystem lie between E1 and E1 + ΔE and
- The energy of second subsystem lie between E2 and E2 + ΔE.
∴ The entropies of the two subsystems are
S1 (E1, V1) = k log Γ1 (E1) and S2 (E2, V2) = k log Γ2 (E2)
- Here Γ1 (E1) and Γ2 (E2) are the volumes occupied by the two ensembles in their Γ space.

- Let the composite system be made up of two subsystems and the microcanonical ensemble of it lie between energy range E and E + 2ΔE
- Also ΔE << E
- In the microcanonical ensemble of composite system
(i) N1 particles whose momenta and coordinate are p1, q1 contained in the volume V1
(ii) N2 particles whose momenta and coordinate are p2, q2 contained in the volume V2
(iii) The energies E1 and E2 of the subsystem satisfy the condition
E < (E1 + E2) < E + 2 ΔE
∴ The total volume of the region of Γ-space corresponding to (i) and (ii) and energy lying between (E1 + E2) and (E1 + E2) + 2ΔE is
Γ1 (E1) Γ2 (E2)
- For total volume of the ensemble Γ (E), we sum of Γ1 (E1) and Γ2 (E2) over E1 and E2.
- For simplicity we take lower bound for both systems to be 0 and divide each of energy spectra into internal of size ΔE, between 0 and E.
- Therefore there are E/ΔE intervals in each spectra.
- Where Ei = energy lying in the centre of each energy internal.
- If the number of particles of composite system N = N1 + N2
- Volume of composite system V = V1 + V2
- Entropy of composite system is

Since ΔE = constant (independent of N)
∴ log (E/ΔE) may be neglected
Conclusion
- Above result proves the extensive property of the entropy.
- The energies of subsystems have the definite values E1 (bar) and E2 (bar) respectively.
- E1 and E2 maximize the function Γ1 (E1) Γ2 (E2), when E1 + E2 < E
∴ δ { Γ1 (E1) Γ2 (E2) } = 0 ; when δE1 + δE2 = 0
or δ log Γ1 (E1) = – δ log Γ2 (E2) ; when δE1 = – δE2
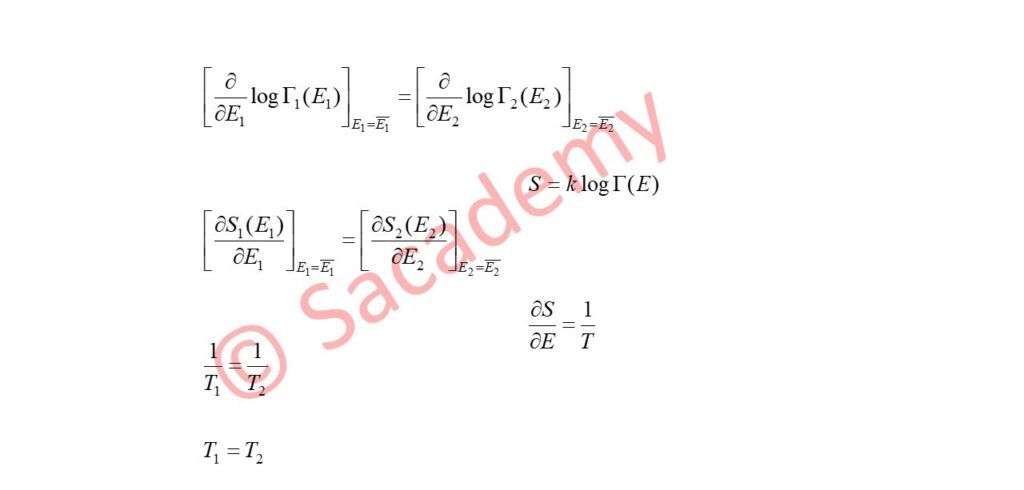
- Thus E1 (bar) and E2 (bar) are such that the two subsystems have the same temperature.
- Thus T is a parameter associated with the condition of equilibrium and it is also related with energy.
- Thus the proof of extensive property of entropy shows that temperature of an isolated system is the parameter representing the equilibrium between one part of the system and another.
- This equation shows that S = k log Γ (E) ; S = k log ω (E) and S = k log Σ (E) are equivalent up to additive constant terms of order log N {O (log N)} or smaller.
S satisfies the properties of the entropies required by the second law of thermodynamics
- In thermodynamics, the entropy (S) is defined only for equilibrium state.
- According to 2nd law of T.D., if an isolated system undergoes a change of thermodynamic state such that the initial and final state are equilibrium state, the entropy of the final state is not smaller than that of the initial state.
∴ Sf ≥ Si
- Let N, V and E are independent macroscopic parameters of a system.
- If system is isolated then N and E are constant.
- Therefore only V can change.
- But V can not decrease, without compressing the system or disturbing its isolation.
- So V can only increase.
- Therefore the second law of TD states that S is a non-decreasing function of V.
- Hence S defined by any of the equation S = k log Γ (E) ; S = k log ω (E) and S = k log Σ (E), represents that S of a system is a function of volume V and internal energy E.
- This represents the connection between the microcanonical ensemble and thermodynamics. as
- To know more about microcanonical ensemble click on the link for English and click on the link for Hindi
Our other websites
https://vacancy.sacademy.co.in
Our YouTube channels