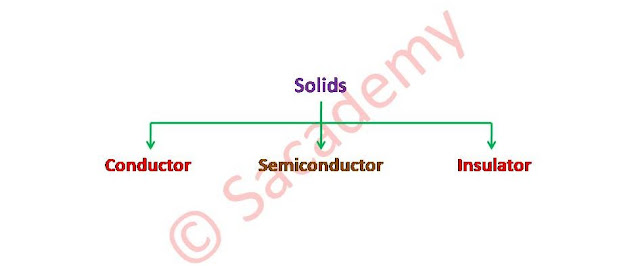
Classification of Solids
- The solids can be classified in three parts, conductor, semiconductor and insulators.
On the electrical point of view
Conductors
- The atoms of conductors have their outermost orbits incomplete initially.
- The valence electrons of conductors are loosely bound to the nucleus, and hence they can be freed from the atoms.
- The electrical and thermal conductivity of conductors are very high.
- In steady state, the conductors obey Ohm’s law.
- The resistance of conductors increases with rise in temperature, it means the temperature coefficient of the conductors are positive.
- Gold, silver, aluminium etc., are the examples of conductors.
Insulators
- The atoms of insulators have their outermost orbits saturated.
- The valence electrons of insulators are tightly bound to the nucleus, and hence they can not be freed from the atoms, or practically they don’t have any free electrons.
- The electrical conductivity of insulators are very small or the electrical resistivity of insulators are very high.
- Glass, mica, quartz, ebonite etc., are some examples of insulators.
Semiconductors
- The atoms of semiconductors have their conductivity intermediate between conductors and insulators.
- The resistivity of the semiconductors are higher than that of the conductors and lower than that of the insulators.
- The conductivity of semiconductors are lower than the conductors and higher than the insulators.
- Resistance and resistivity of these substances decreases with rise in temperature, it means the temperature coefficient of the semiconductors are negative.
- The conductivity of semiconductors can be increased by adding some impurities in it.
- Ge, Si, etc., are the examples of semiconductors.
On the basis of energy bands
- The electrical properties of the materials can be explained by energy bands.
- An electron in a solid can have only those discrete energies that lie within these energy bands, such bands are known as allowed energy bands.
- The allowed energy bands are separated by some gaps in which there is no allowed energy bands, such gap is known as forbidden energy bands.
- The energy bands occupied by valence electrons are known as valence band, and the energy bands occupied by conduction electrons are known as conduction band.
- The gap between valence band and conduction band is energy gap or forbidden band.
- Higher is the energy gap, lower is the electrical conductivity of the material.
Conductors
- The difference between valence band and conduction band in conductors are zero, or both the bands in conductors overlap.
- There are sufficient amount of free electrons in conduction band in conductors.
- In conductors, the space for moving, the free electrons is very small, or there is no free space to move.
- The conductivity of conductors is very large at room temperature, and as the temperature increases their conductivity decreases.
Insulators
- In insulators, the valence band is completely filled, and the conduction band is completely empty.
- In insulators, the difference between valence band and conduction band is very large.
- It is found that with rise in temperature, the conductivity of insulators can be increased.
Semiconductors
- The semiconductors has a partially filled conduction band and partially filled valence band.
- The energy gap between valence band (V.B.) and conduction band (C.B.) in semiconductors are very small (about 1 eV).
- At absolute zero temperature the semiconductor behaves as insulators.
- With rise in temperature the conductivity of semiconductor increases, or resistivity decreases.
- At very high temperature the semiconductor behaves as conductor.
![]()
Energy band diagram of solids
To know in detail about the classification of solids please click here.