Gas laws on the basis of kinetic theory
Boyle’s law
- At a constant temperature the product of the pressure and volume for a given gas is always constant.
- From kinetic theory of gas
- If T remains constant, then average kinetic energy of molecule remains constant.
- In a given volume the number of molecule of gas remains constant.
PV = constant
- This is called Boyle’s law
Charles’ law
- At constant pressure the volume of any gas is directly proportional to its absolute temperature.
- From kinetic theory of gas

- Thus if P = constant, then
V ∝ T
- This is called Charles’ law.
Gay Lussac’s law
- At constant volume, the pressure of a gas is directly proportional to the absolute temperature.
- Perfect gas equation
PV = RT
or P = (R / V) T
- If V = constant, then V ∝ T
- This is called Gay Lussac’s law.
Avogadro’s law
- At constant temperature and pressure, equal volume of different gases have equal number of molecules.
- Let m1, n1 and c1 are mass per molecule, number of molecules per c.c. and r.m.s. velocity of one gas respectively.
- And m2, n2 and c2 are mass per molecule, number of molecules per c.c. and r.m.s. velocity of second gas respectively.
- If two gases exert the same pressure P, then since P = (1/3)mnc2
- If two gases are at same temperature, then their mean K.E. will be same
- It means equal volume of two gases at the same temperature and pressure have the same number of molecules, which is Avogadro’s hypothesis.
Dalton’s law of partial pressure
- If a large number of gases available in a container do not chemically reacted, then the total pressure of the gas will be equal to the partial pressure of individual gases.
- Let there are number of gases
- Densities of the gases are ρ1, ρ2, ρ3, …
- Mean square velocities of the gases are c12 (bar), c22 (bar), c32 (bar), …
- If these gases mixed up in the same volume, then resultant pressure
- Here P1, P2, P3, … are the pressures, which each gas would exert it along occupies the whole volume.
- This is called Dalton’s law of partial pressure.
Graham’s law of diffusion
- According to it the rate of diffusion of a gas is inversely proportional to the square root of its density.
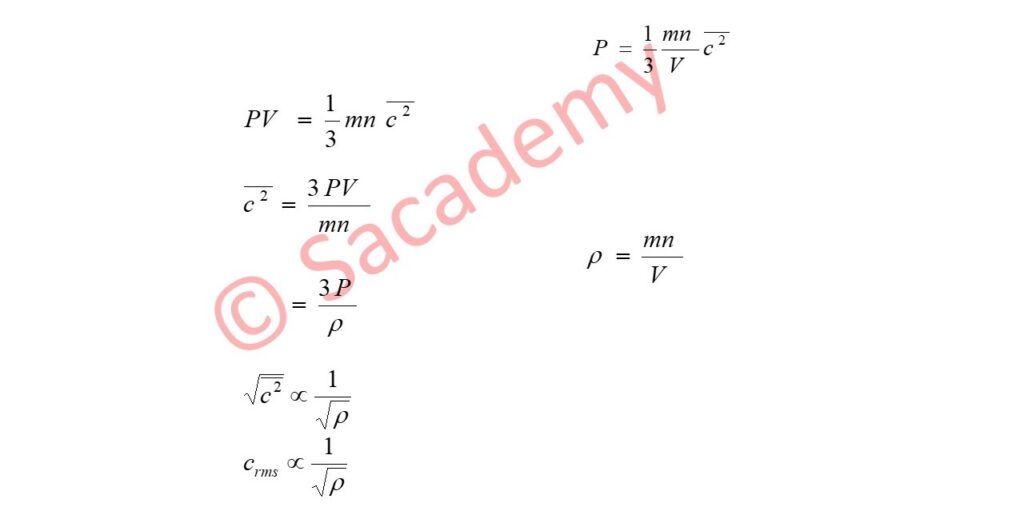
- According to Maxwell the average velocity of molecules is directly proportional to the root mean square velocity of them.
- But crms ∝ 1 / √ ρ …
- Thus the average velocity of molecules is inversely proportional to the square root of density of gas.
- Since the rate of diffusion of any gas depends on the average velocity of molecules.
- Therefore the rate of diffusion of any gas is inversely proportional to the square root of the density of gas.
- This is called Graham’s law of diffusion.
To know in detail about Gas laws on the basis of kinetic theory click here.
Our other websites
Our YouTube channels