Gibbs paradox
- The entropy of an ideal gas

- Now, we consider two ideal gases with N1 and N2 particles respectively.
- These two gases are in two separate container of volumes V1 and V2 at the same temperatures i.e., T1 = T2 = T and having same density.
- Now, the gases are allowed to mix in a volume V = V1 + V2, then the entropy of the composite system (the temperature remain constant) is given by
S = S1 + S2
= N1k [log V1 + 3/2 log T + C] + N2k [log V2 + 3/2 log T + C]
- If the particles in the two systems are the same and for convenience we take V1 = V2 = V, and N1 = N2 = N, then total entropy of the system

- This equation shows that by joining two moles of two different gases by removing a partition between them, the entropy of the system increases by an additional factor 2Nk log 2.
- This is called Gibbs paradox.
Explanation

- After the removal of partition, a molecule of each system, being indistinguishable and they can be found anywhere.
- Mixing of two gases within the volume 2V instead of V and hence external parameter become 2V and therefore the possible states of energy are to be calculated with the total volume 2V instead of V.
- This explains the occurrence of the additional factor 2Nklog2.
- But if we take two systems as the same (two molecules of the same gas), then the molecules will be indistinguishable (same as in quantum mechanics).
- In this case we have to correct the formula of entropy by using the idea of indistinguishability to classical approximation.
- If there are n indistinguishable molecules in a system, then the correct formula of entropy is

- Therefore the entropy of the system
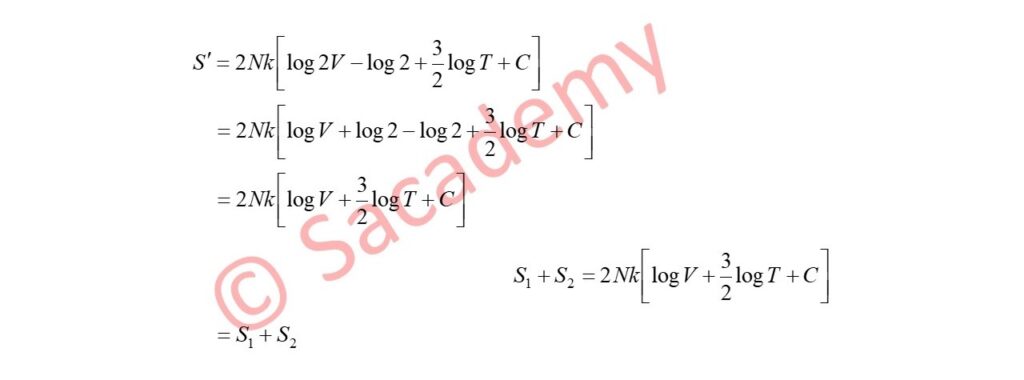
- Gibb’s paradox is thus resolved by the concept of quantum mechanics.
- To know in detail about Gibbs paradox click on the link for English and click on the link for Hindi
Our other websites
https://vacancy.sacademy.co.in
https://vacancy.sacademy.co.in
Our YouTube channels


I couldn’t resist commenting. Verry well written! http://boyarka-inform.com/