Maxwell Boltzmann Statistics
- It is applied to distinguishable particles.
- Particles are distinguishable from each other.
- Each cell may contain 0, 1, 2, … ni particles.
- Total number of particles of system remain constant, n = Σni = constant
- Sum of energies of all the particles in the different groups taken together i.e., total energy of the system remain constant E = Σniεi = constant
- Consider a system of n distinguishable particles.
- These particles be divided into groups or levels such that
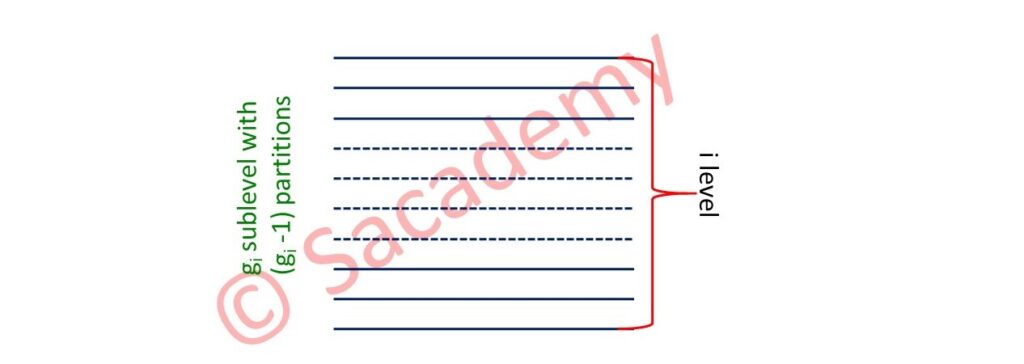
- Energy levels ε1, ε2, ε3, …εi
- Degeneracies g1, g2, g3, …gi
- Occupation number n1, n2, n3, …ni
- Consider a box, divide it into gi sections, distribute ni particles among them.
- Number of ways to distribute n1 particles in first state are
- Number of ways to put n2 particles in the second state are
… … … … … …
- Total ways to distributions
- First particle can be accommodated in any of the gi group by gi ways.
- Since there is no restriction, so second particle can be accommodated in gi group by gi ways.
… …. …. …. ….
- Thus ni particle can be accommodated in gi group by gini
- Total number of eigen state for the whole system
- According to the postulates of a priori probability of eigen state
- Sterling approximation log x! = x log x – x
- For maximum probability, δ log ω = 0
- Other condition
n = Σ ni = constant
or δn = Σ δni = 0 …(2)
and E = Σ ni εi = constant
or δE = Σ εiδni = 0 …(3)
- Lagrange’s method of undetermined multiplier, (1) + (2) × α + (3) × β
Σ [{log (ni / gi ) + 1)} + α + βεi ] δni = 0
- But δni is arbitrary
∴ log (ni / gi ) + 1 + α + βεi = 0
or log (ni / gi ) + α + βεi = 0
or log (gi / ni ) = α + βεi
or (gi / ni ) = exp (α + βεi )
or ni = gi / exp (α + βεi )
To know more about Maxwell-Boltzmann statistics in detail click here for English and click here for Bilingual (Hindi/English)