X-ray spectra
X-rays
- X-rays were discovered by a German physicist Wihelm Conrad Roentgen in 1895 during the study of the properties of cathode rays.
- These rays are actually electromagnetic rays of lower wavelengths having range from 10 Å to 0.5 Å.
- Higher wavelength side X-rays are soft X-ray and the lower wavelength side X-rays are hard X-ray.
Properties of X-rays
- Since the X-rays do not deflect by electric and magnetic fields, hence they does not consist of charged particles.
- X-rays are electromagnetic radiation of very short wavelength.
- X-rays affect photographic plate like light, but their effect is much more intense than light.
- X-rays travel in straight lines with the velocity of light.
- They produce fluorescence in several materials.
- X-rays ionize the gas through which they pass.
- X-rays have high penetrating power and can pass through many solids.
- They produce photo electric effect also.
- X-rays also show interference, diffraction and polarization similar to the light rays.
X-ray spectra
- The wavelength of X-rays can be determined by Bragg’s law.
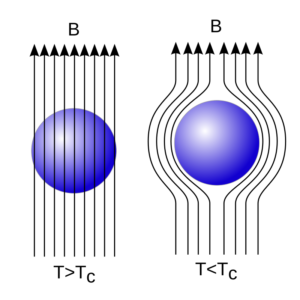
- If we plot a graph between wavelength of X-rays obtained from any source and their intensity then the graph will be according to figure given above.
- Three waves are shown using Rh (Rhodium) as a target and at different potential differences.
- It is clear from spectrum that at every stage, spectrum is continuous and obtained at a particular wavelength.
- If applied potential difference is below 23 kV, then only continuous emission spectra is obtained.
- But at higher potential difference a line spectrum is also overlapped with a continuous spectrum.
- A line spectra gives the information about the target nuclei.
- Thus the spectrum from an X-ray tube contains two parts:
Continuous spectrum
- It consists of all possible wavelengths, from a certain lower limit to higher values continuously, like a visible spectrum. So it is also known as white spectrum.
Characteristic or line spectra
- It consists of definite well defined wavelengths superimposed on the continuous spectrum.
- The spectral lines of this spectrum generally occurs in small groups.
- These lines are characteristics of the material of the target nuclei.
Continuous X-ray spectrum
- The continuous X-ray spectrum was discovered by Duane and Hunt.
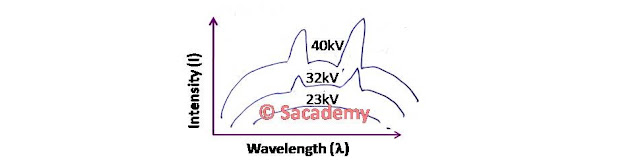
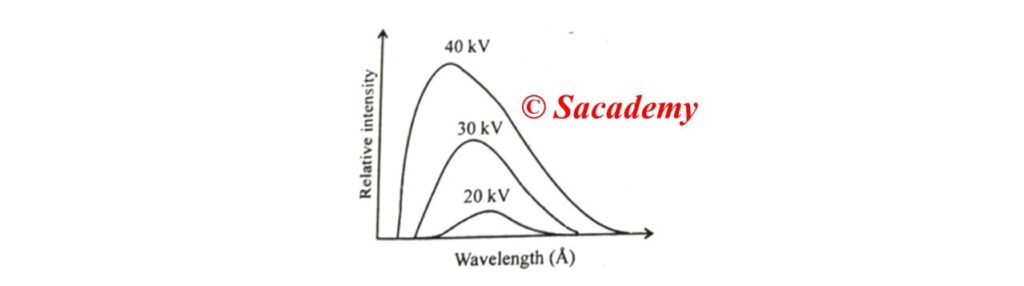
- If by taking tungsten element as a target nuclei and by applying different potential difference on the X-ray tube, we plot the intensities of the rays produced against wavelength, then the curve will be according to the figure.
- This curve provide the continuous X-ray spectra.
- For each potential difference the intensity-wavelength (I-λ) curve starts at a particular wavelength, rises rapidly to a maximum and then drops gradually.
- The wavelength at which the intensity become maximum depends on accelerating voltage (Va). Higher the value of accelerating voltage, higher will be the intensity.
- The peak intensity shift towards the shorter λ side as the value of Va increases. Thus the penetration power of X-rays increases with the voltage.
- For each anode potential, there is a minimum wavelength (λmin) below which no radiation is emitted. Its value depends on the target nuclei and above this critical value, the intensity of radiation increases.
- By applying different potential difference at X-ray tube, we get continuous spectrum for every wavelength.
- The total power of X-rays (P) depends on the area of experimental wave.
- The total power is directly proportional to the square of the applied voltage.
P ∝ V2
- The total power is directly proportional to the atomic number of target (Z).
P ∝ Z
or P ∝ ZV2 ⇒ P = kZV2
- Here k is proportionality constant
- When the voltage across the X-ray tube is increased, λmin shifted towards small value.
∴ λmin ∝ 1/V
or νmax ∝ V
or νmax = kV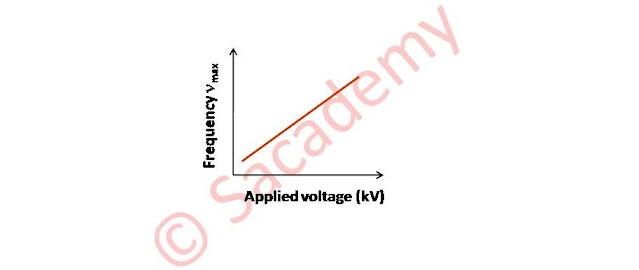
Origin of continuous X-ray spectrum
Duane and Hunt’s law
- If a high energy electron beam incident on a target nuclei, then it enters into the target and deviates from its actual path.
- In this process the loss of energy of incident electron beam provides an X-ray photon and electron loses its velocity due to interaction with strong field of target.
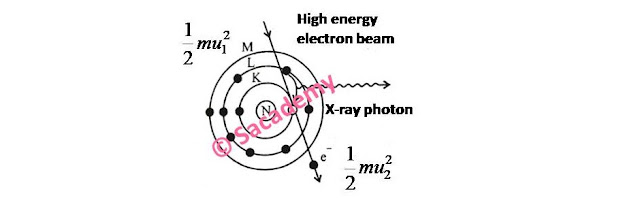
- If u1 and u2 be the initial and final velocities of electron, then
- Energy of X-ray photon, hν = ½ mu12 − ½ mu22
- If electron stops into the target, then u2 = 0, ν = νmax
- hν = mu12
- eV = hνmax
- eV = ch / λmin (∵ c = νλ)
- λmin = ch / eV
- This is Duane and Hunt’s law.
- λmin = (12400 / V) Å
- Most of the electron that generate X-ray photon give up only a part of their energy in this process.
- Therefore most of the X-radiation is of longer wavelength than λmin.
- Thus continuous spectrum is the result of the inverse photo electric effect.
To get the detailed knowledge about X-rays click here.